NE HEALTH BUREAU
NEW DELHI, JAN 9
Takeda Biopharmaceuticals India Private Limited (formerly known as Baxalta Bioscience India Pvt. Ltd.), a global values-based, R&D-driven biopharmaceutical leader has recently launched CINRYZE, an innovative injectable prescription medicine for the treatment of hereditary angioedema (HAE) patients. With eight years of global clinical experience proving efficacy and safety, CINRYZE has the potential to mark a breakthrough in the episodic treatment, short and long-term prophylaxis for HAE. Moreover, CINRYZE is the pioneer C1 esterase inhibitor (C1-I NH) approved by the FDA & EMA for the symptomatic management of HAE and for preventing future angioedema attacks.
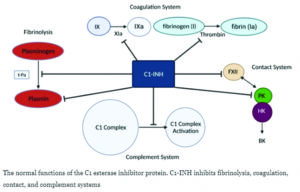
- CINRYZE is plasma-derived C1-I NH approved for routine prevention (prophylaxis), short-term prevention or pre-procedure prevention, and acute attacks of HAE.
- Among adult HAE patients, 51% miss at least one day of work (mean 3.3 days), 44% students miss at least one day of school (mean 1.9 days) and 59% miss at least one day of leisure activities (mean 3.3 days) as result of their most recent HAE attacks.
- With strong clinical evidence, CINRYZE™ is proven to reduce the frequency and severity of attacks with fixed dosing regimen.
CINRYZE is indicated in India for:
- Routine prevention (prophylaxis) of angioedema attacks in adults, adolescents and children 6 years of age and above with HAE[3].
- Treatment of angioedema attacks and pre-procedure prevention of angioedema attacks in adults, adolescents and children 2 years of age and above with HAE [3].
Speaking at the launch, Serina Fischer, General Manager, Takeda Biopharmaceuticals India Private Limited said, “At Takeda, we are committed to bringing innovative treatment to fulfil the unmet medical needs across our core therapy areas. We are confident that the launch of Cinryze will bridge the gap in the treatment of HAE patients in India. The launch furthers our commitment towards rare diseases patients in India.”
Sony Paul, Franchise Head, Rare Diseases, Takeda Biopharmaceuticals India Private Limited added, “As per data, there are likely to be more than 30,000 undiagnosed patients at present in the country. These patients are suffering due to the lack of diagnosis and treatment. With the launch of Cinryze, our aim is to reduce acute attacks of HAE and support the prevention of HAE attacks by prophylactic regime thus improving the quality of life of HAE patients.”
Hereditary Angioedema (HAE) is a rare genetic condition that causes swelling in different parts of the body like limbs, face, abdomen, and larynx. HAE is caused by a mutation in the gene which produce protein called the C1 esterase inhibitor leading to its reduced level or compromised functioning. Symptoms of HAE often present in childhood, and while attacks can occur at any age, early onset may predict a more severe disease course. Attacks often occur in children without a clear trigger,[5] and may affect a child’s participation in school, activities, and sports, which can leave them feeling socially isolated [7,8]. It can be life-threatening in severe cases in which the swelling attacks can manifest in the larynx (voice box), or trachea (windpipe).
The disease is usually identified by recurrent episodes of fluid accumulation outside the blood vessels, causing rapid swelling of body tissues. The symptoms of HAE could be like an allergic reaction; however, the potential outcome could be life-threatening in case of HAE. Due to the lack of awareness among healthcare professionals, HAE is highly underdiagnosed in the country.












